Research
Living organisms derive most of their free energy from oxidation-reduction (redox) reactions, i.e. processes involving transfer of electrons. To accomplish this, they use enzymes, oxidoreductases, which catalyze individual chemical redox reactions. These enzymes embed redox active cofactors, such as hemes and iron-sulfur clusters, and are often assembled into chains that perform specific biological functions. How these systems are designed to secure both the recognition of the reacting partners and catalysis is central to our understanding of the nature of their energetic efficiency and physiological regulation. It now becomes clear that more or less severe dysfunctions of bioenergetic enzymes are associated with increased vulnerability to many types of side reactions, including those commonly associated with production of damaging reactive oxygen species (ROS). This can create conditions predisposing towards or resulting in various diseases, such as several neurodegenerative and metabolic disorders, aging and aging-related diseases, and cancer.
Our research seeks to expose the molecular mechanisms of efficient energy conversion in cytochrome bc1 (mitochondrial complex III), a key enzyme of mitochondrial respiration and many other electron transport chains (including bacterial and photosynthetic chains). This multi-subunit and multi-cofactor protein complex connects functionally a pool of ubiquinones in membranes with a pool of cytochromes c outside membranes. In doing so, it uses hydroquinone/quinone redox chemistry to translocate protons across bioenergetic membranes to build up protonmotive force that powers cellular ATP synthesis. It appears be an important point of regulation of bioenergetics systems.
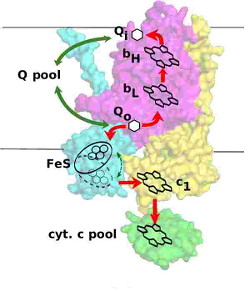 |
| Simplified scheme of electron transfer in cytochrome bc1 |
The catalytic cycle of cytochrome bc1, known as the Q cycle, takes place within the two chains of cofactors that embed two catalytic quinone oxidation/reduction sites, the Qo and the Qi site. The c-chain consists of the iron-sulfur (FeS) center, heme c1 and cytochrome c; the b-chain consists of heme bL, heme bH and quinone at the Qi site. Those two chains are linked through the operation of the Qo site, which reversibly oxidizes quinol by delivering one electron into the FeS center and the other into the heme bL.
The energetic efficiency of cytochrome bc1 relies on this unique in biology bifurcation reaction which tightly couples electron transfer in two chains. Despite a large energetic gap between the high-potential components of the c-chain and the low-potential components of the b-chain, that meet at the quinone binding catalytic Qo site, one electron must exclusively travel along the c-chain while the other travels exclusively along the b-chain. Any reaction that breaks this rule and allows direct electron tunneling between the chains results in energy-wasting short circuit.
We want to understand the molecular engineering of these two cofactor chains and the catalytic Qo and Qi sites and their natural design to suppress damaging reactive oxygen species (ROS) and short circuits. Towards this goal we examine structural and thermodynamic elements of cytochrome bc1 function, as well as parameters of failure and conditions enhancing side reactions.
Role of structural symmetry of cytochrome bc1 and mechanisms of catalytic reactions and short-circuit supression.
In membranes, cytochrome bc1 assembles as a homodimer. Structurally, spectroscopically and electrochemically symmetric monomers form an extraordinary H-shaped electron transfer system that distributes electrons between four catalytic quinone oxidation-reduction sites. This raises several mechanistic questions about the operation of the dimer and its parts and regulation of electron flow. To address these questions we exploit a flexibility of bacterial photosynthetic system with which we can modify properties of individual cofactors and the catalytic sites by site-directed mutations and also break symmetry of the dimer. Modified complexes are analyzed by a variety of methods; including time-resolved optical spectroscopy and EPR.
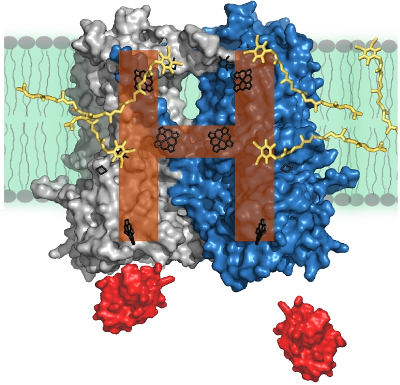
H-shaped electron transfer system of dimeric cytochrome bc1
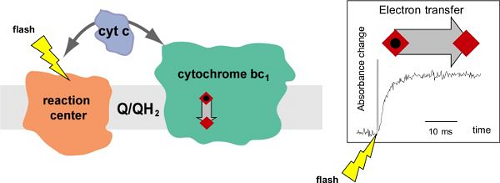
Light-induced electron transfer in photosynthetic bacterial model
Mechanisms of molecular recognition and inter-protein electron transfer.
At present, we can appreciate many of the structural and kinetic details about the interactions between specific redox proteins. However the meaning of dynamic equilibrium between the various steps of molecular association in the context of the mechanism of electron transfer is not as well understood. Two large-scale protein motions are involved in cytochrome bc1 catalysis: interaction of cytochrome c1 subunit with soluble cytochrome c and the FeS head domain movement between the cytochrome b and cytochrome c1 subunits. We examine those interactions using EPR techniques.
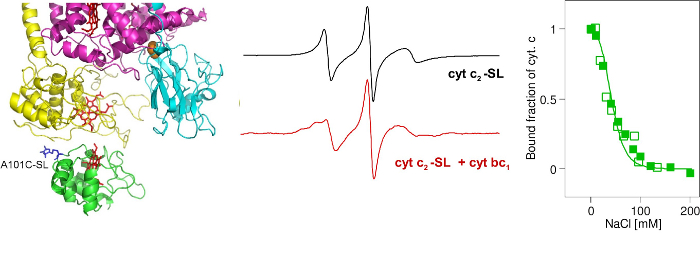
Monitoring binding of cytochrome c with cytochrome bc1 using spin-labeled cytochrome c.
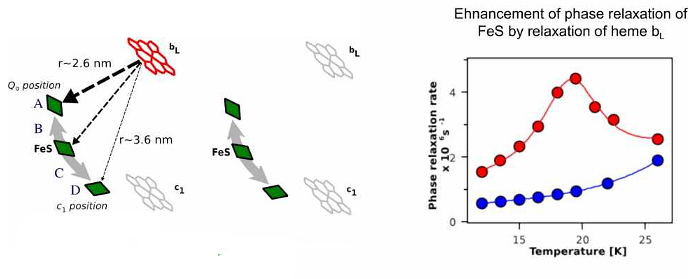
Monitoring changes in FeS position through magnetic interactions between heme bL and FeS.
Parameters of failure and mechanism of ROS production.
Superoxide is commonly thought to be a product of an oxidation of a highly unstable semiquinone formed in the Qo site (SQo), as a side reaction of the Q cycle. However, the specific reactions that lead to superoxide generation remain poorly understood. There is an ongoing debate as to whether cytochrome bc1 does produce superoxide in living cells and if so under what conditions and with what mechanism. We use modified cytochrome bc1 with knockout mutations that introduce specific rate limiting steps to manipulate the electron flow through the chains and isolate states linked with superoxide production.
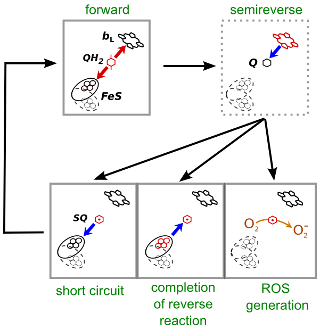
Model of superoxide generation in the Qo site of cytochrome bc1.
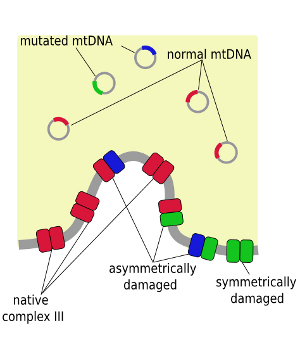
Molecular basis of mitochondrial mutations.
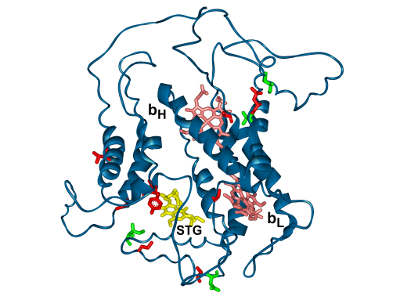
In the era of rapidly growing "molecular mitochondrial medicine" and "evolutionary medicine", new mitochondrial DNA mutations are being identified every year. While they often are proposed to confer either adaptive or pathogenic effect for physiology, little is known how such mutations affect the work of the mitochondrial enzymes on molecular level. This includes reversible operation of cytochrome bc1. We mimic mitochondrial mutations of cytochrome b in bacterial photosynthetic model system to examine their structural, kinetic and energetic effects on cytochrome bc1 function.
 |
 |
|
Some of pathogenic (red) or adaptive (green) mutations in the vicinity of the Qo or Qi site. |
Symmetrically and asymmetrically damaged cytochrome bc1 upon occurrence of mutations in mtDNA. |

